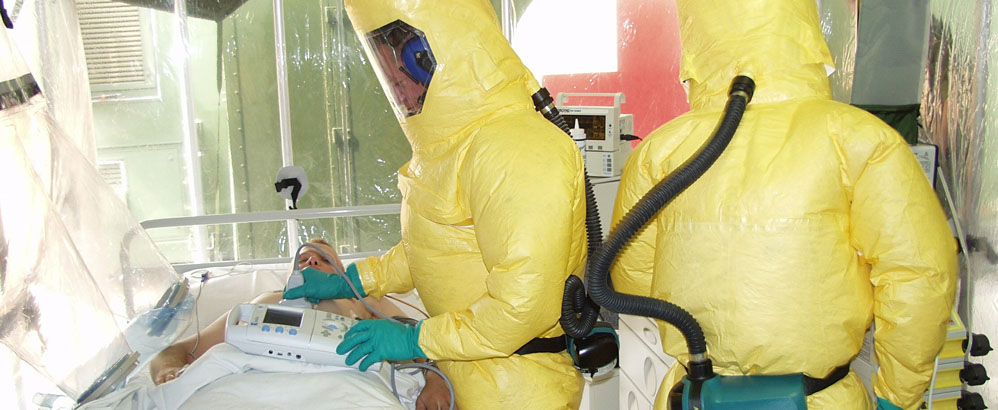
According to a press release issued by Università della Svizzera italiana (USI: University of Italian Switzerland), the FDA has approved a drug for use in the USA to treat Ebola which was originally developed on the back of research conducted at two labs based in the canton of Ticino: the Institute for Research in Biomedicine (IRB), which forms part of USI, and Humabs BioMed. Both facilities are located in Bellinzona. Humabs is a subsidiary of Vir Biotechnology, which is headquartered in San Francisco, California.
The drug is based on the monoclonal antibody mAb114. The IRB was able to isolate and characterize this antibody in collaboration with Humabs. This is the first form of treatment based on just a single antibody developed to combat the effects of the deadly Ebola virus.
The results obtained by the Ticino labs and subsequent publication in highly regarded scientific journals such as “Science” and “The Lancet” contributed to the success of the clinical field trials conducted in 2018 during a renewed outbreak of the virus in the Democratic Republic of Congo, the press release explains. Furthermore, the “significant support” of the U.S. Defence Advanced Research Projects Agency (DARPA) over several years was also key to this successful outcome.
Related news
Contact us
Can we put you in touch with a peer company or research institute? Do you need any information regarding your strategic expansion to Switzerland's technology and business center?
info@greaterzuricharea.com